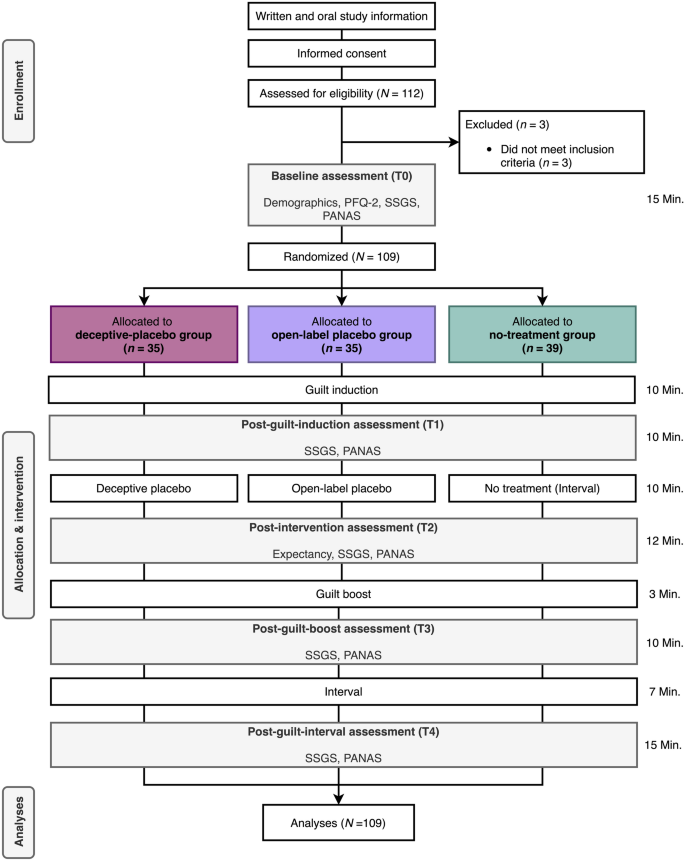
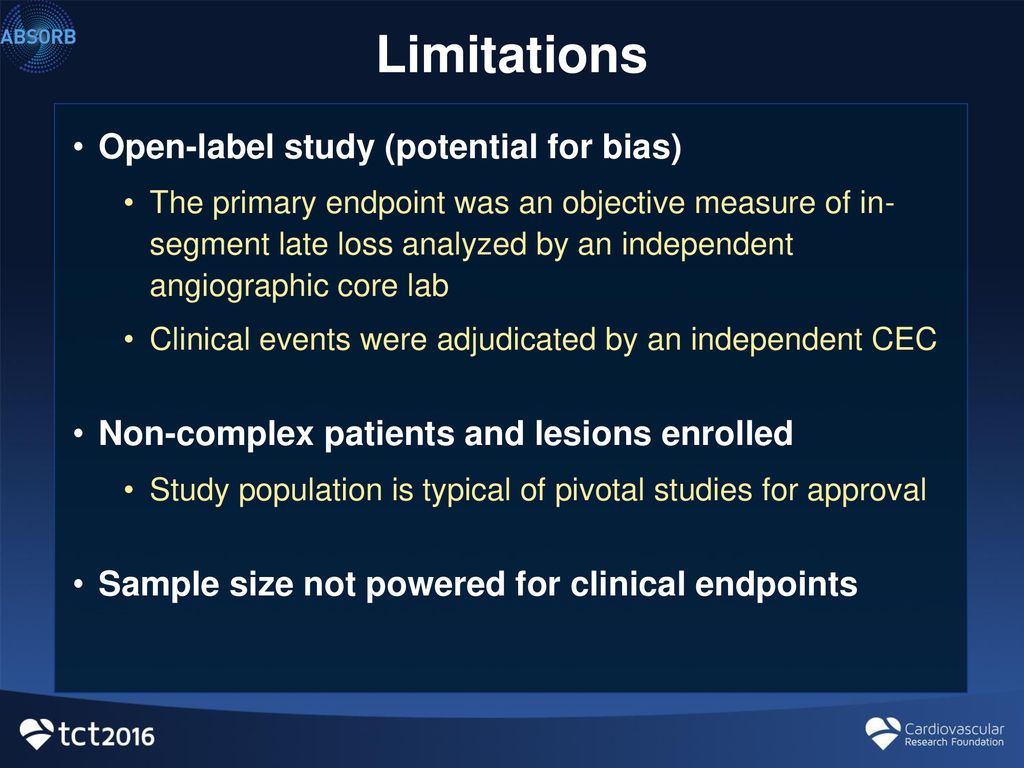
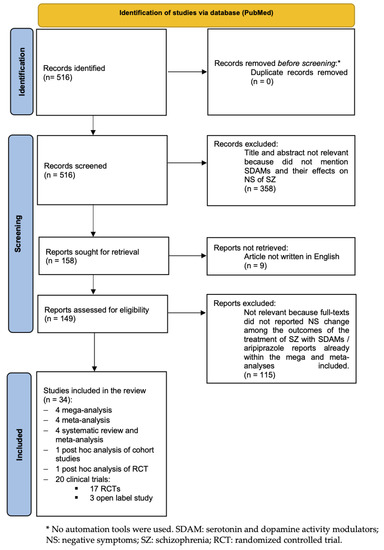
44 open-label study bias
National Center for Biotechnology Information National Center for Biotechnology Information Bias was reduced in an open-label trial through the removal of ... Bias was reduced in an open-label trial through the removal of subjective elements from the outcome definition Modifying the outcome definition to exclude subjective elements substantially reduced bias. This may be a useful strategy for reducing bias in trials that cannot blind outcome assessment.
PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG Even for open label studies, it's still desirable blind the study sponsor to reduce potential bias due to the sponsor knowing the treatment level aggregated data while the study is ongoing. The practice helps increase credibility of study results and thus should be followed, particularly for registration studies. In open label studies, in ...

Open-label study bias
(PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India.... External and internal validity of open label or double-blind trials in ... to the trial design of open-label vs. double-blind double-dummy (Table 1). For instance, while dabigatran was tested in AF using an open-label study [1] and in acute deep vein thrombosis (DVT) and pulmonary embolism (PE) using a double-blind double-dummy trial [2], rivaroxaban was tested inversely with open-label trials in acute DVT and PE [3 ... Investigating the impact of open label design on patient‐reported ... Bias may occur in open-label trials, as observer bias and disappointment bias. 34 - 37 Therefore, according to the FDA, patients may be prone to provide biased reports of their own symptoms if they are aware of the treatment they received and lead to an overestimation of the treatment difference observed between the two treatment arms.
Open-label study bias. Bias for Patient-Reported Outcomes in Open-Label Cancer Trials: How Big ... A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient's knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology. Reducing bias in open-label trials where blinded outcome assessment is ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [ 6 - 10 ]. Reducing bias in open-label trials where blinded outcome ... - PubMed Reducing bias in open-label trials where blinded outcome assessment is not feasible: strategies from two randomised trials Authors Brennan C Kahan 1 , Suzie Cro , Caroline J Doré , Daniel J Bratton , Sunita Rehal , Nick A Maskell , Najib Rahman , Vipul Jairath Affiliation A potential bias in safety evaluation during open-label ... - PubMed A potential bias in safety evaluation during open-label extensions of randomized clinical trials The confounding described here is not prevented by randomization because it develops in a non-randomized add-on analysis to the trial. The bias can be removed, however, by controlling for time since randomization in the analysis of the data.
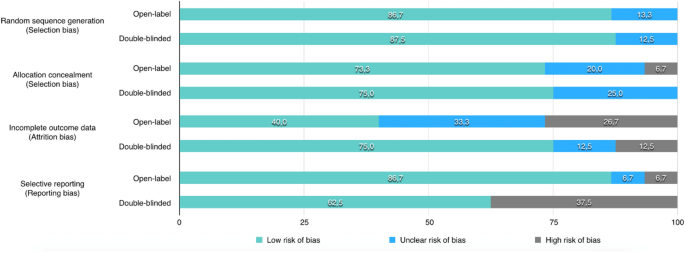
Open-label extension studies: do they provide meaningful ... - PubMed Some increased confidence about incidence rates might result from the open-label extension study; however, as these studies are essentially uncontrolled and biased, the data are not of great value. Other benefits have been proposed to be gained from open-label extension studies. 8.4 Introduction to sources of bias in clinical trials - Cochrane Detection bias refers to systematic differences between groups in how outcomes are determined. Blinding (or masking) of outcome assessors may reduce the risk that knowledge of which intervention was received, rather than the intervention itself, affects outcome measurement. External and internal validity of open label or double-blind trials in ... In these trials, open-label or double-blind double-dummy designs are being used to evaluate the efficacy and safety in prevention and treatment of venous thromboembolism or stroke prevention in atrial fibrillation in several thousands of patients. Design characteristics, risk of bias, and reporting of randomised ... In trial CA204-009, which was open label, outcome assessors were aware of the intervention received by study participants, and assessment of the progression free survival outcome could have been influenced by knowledge of the intervention received. ... Furthermore, our risk of bias assessments were not blinded to study results because risk of ...
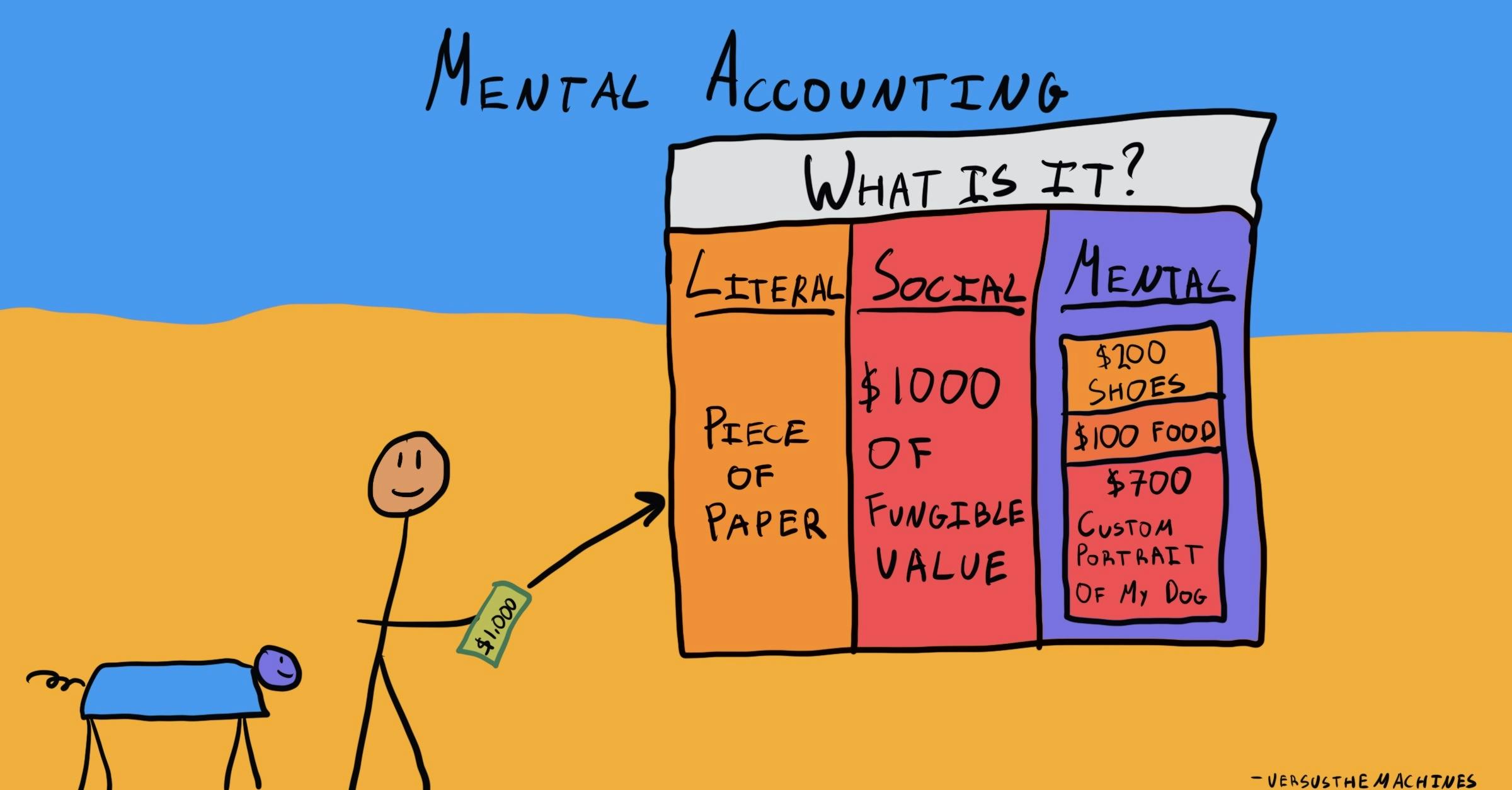
Exploring open-label bias in patient-reported outcome (PRO) emotional ... e18702 Background: Concern exists that patients' self-reports may be biased in open-label trial designs. We compared PRO emotional domain results between investigational arms of paired open label and double-blind trials of the same drug and disease population. We hypothesized that greater improvement in emotional domain scores would be found in the investigational arms of open label compared ... What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities. PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized Effects of open-label placebos in clinical trials: a ... - Nature Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. ... First, we detected hints of a publication bias in the study sample ...
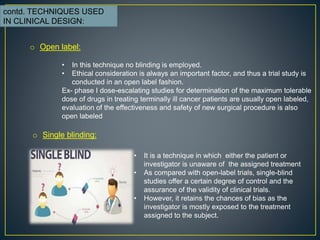
Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a key...
Investigating the impact of open label design on patient‐reported ... Bias may occur in open-label trials, as observer bias and disappointment bias. 34 - 37 Therefore, according to the FDA, patients may be prone to provide biased reports of their own symptoms if they are aware of the treatment they received and lead to an overestimation of the treatment difference observed between the two treatment arms.
External and internal validity of open label or double-blind trials in ... to the trial design of open-label vs. double-blind double-dummy (Table 1). For instance, while dabigatran was tested in AF using an open-label study [1] and in acute deep vein thrombosis (DVT) and pulmonary embolism (PE) using a double-blind double-dummy trial [2], rivaroxaban was tested inversely with open-label trials in acute DVT and PE [3 ...
(PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India....



























Komentar
Posting Komentar